Introduction: Patients with histological transformation (HT) of Waldenström macroglobulinemia (WM) who relapse or are refractory (R/R) have a poor prognosis with standard chemoimmunotherapy, notably patients who are unable to undergo high dose chemotherapy (HCT) and autologous stem cell transplantation (ASCT) or who relapse after HCT/ASCT. CD19-targeted chimeric antigen receptor (CAR) T-cell therapies can lead to durable responses in relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and thus may represent a promising therapeutic option in R/R transformed WM (tWM); however, data from pivotal trials is lacking. This study aimed to evaluate the efficacy and safety of anti-CD19 CAR T-cells in real-word patients with R/R tWM.
Methods: We retrospectively identified patients with biopsy-proven transformed WM/lymphoplasmacytic lymphoma treated with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) in the French DESCAR-T registry (NCT04328298) and 2 US centers. The primary endpoint was best complete response rate (CRR). Secondary outcomes were best overall response rate (ORR), progression-free survival (PFS), overall survival (OS) and safety. Responses were assessed according to the Lugano 2014 criteria at 1 month, 3 months and 6 months. Subsequent assessments were per institutional pratice. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded according to the ASTCT 2019 consensus criteria. Hematological toxicity and infections were graded according to the NCI CTCAE (version 5.0).
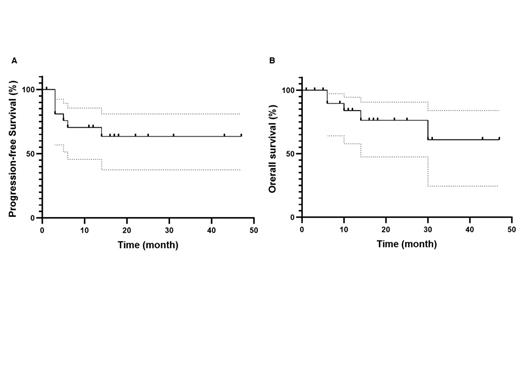
Results: Between June 2017 and May 2023, 22 patients (18 from DECAR-T centers and 4 from US centers) with R/R tWM were treated with anti-CD19 CAR T-cells (13 axi-cel and 9 tisa-cel). Median age at WM diagnosis was 58 years (range 35-72). MYD88 L265P and CXCR4 mutations were present in 85% (11/13) and 29% (2/7) of patients with available data, respectively. Patients received a median of 1 prior line of treatment (range, 0-4) for WM and 2 prior lines (range, 1-4) for HT. Eight patients had received prior ASCT (2 for WM and 6 for HT) and 1 patient allogeneic SCT. The median time from WM to HT diagnosis was 4.5 years (range, 0-32) and 26 months (range, 3-90) from HT diagnosis to CAR T infusion. Of note, CNS involvement was present in 3 patients at DLBCL relapse before CAR T-cell therapy. At time of infusion, the median age was 66 years (range, 41-82) and 8 patients (36%) were older than 70 years. Median Eastern Cooperative Oncology Group (ECOG) was 1 (range, 0-2). Bridging therapy was administered to 16 patients (73%). Following bridging therapy, 6 patients had progressive disease (PD), 4 stable disease (SD) and 6 were responders (2 complete responses (CR) and 4 partial responses (PR)). After CAR T-cell infusion, best CRR was 82% and best ORR was 95%. The ORR/CRR was 95%/77% at 1 month, 76%/71% at 3 months and 68%/68% at 6 months. Seventeen patients (78%) experienced CRS, including 2 grade 3 (all after axi-cel), and 9 ICANS occurred (41%), including 1 grade 3 and 1 grade 4 (all after axi-cel). Eight patients (36%) presented infections, including 3 grade 3 and 1 grade 4. Neutropenia was reported in 78% (grade 3-4 = 68%), thrombocytopenia in 86% (grade 3-4 = 45%) and anemia in 86% (grade 3-4 = 32%) of patients. After a median follow-up of 17 months (range, 1-47), the 1-year PFS and OS were 70% and 84% respectively (figure). Five deaths were reported, 4 due to progressive disease and 1 from SARS-CoV-2 infection.
Conclusion: This study shows a high efficacy of anti-CD19 CAR T-cell therapy in R/R tWM with no unexpected toxicity. Longer follow-up is needed to confirm the long-term efficacy of CAR T-cells in tWM.
Disclosures
Di Blasi:BMS: Consultancy; abbvie: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Kite Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy. Cheminant:Amgen: Honoraria; Innate Pharma: Research Funding; AstraZeneca: Other: Travel accomodations and Meeting inscription; Abbvie: Research Funding. Gros:Gilead: Consultancy, Other: Travel and accommodation expenses; Milteny: Consultancy; BMS: Consultancy; Novartis: Consultancy, Other: Travel and accommodation expenses. Bachy:Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Takeda: Honoraria; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Incyte: Honoraria; Novartis: Honoraria, Other: Personal Fees; Amgen: Research Funding; Roche: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees. Treon:Eli Lilly: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Bristoll Myers Squibb: Consultancy, Research Funding; Abbvie/Pharmacyclics: Consultancy, Research Funding. Reshef:Takeda: Consultancy, Honoraria, Research Funding; Atara Biotherapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Gilead Sciences: Consultancy, Honoraria, Other: Travel support, Research Funding; J&J: Research Funding; Jasper: Consultancy; Capstan: Consultancy; MidaTech: Consultancy; Orca: Consultancy; Synthekine: Consultancy, Research Funding; CareDx: Research Funding; Precision Biosciences: Research Funding; Bayer: Consultancy; TScan: Consultancy, Research Funding; Regeneron: Consultancy; Instil Bio: Consultancy; Incyte: Consultancy, Research Funding; Allogene: Consultancy; Quell Biotherapeutics: Consultancy; Sanofi: Research Funding; Immatics: Research Funding; TCR2: Research Funding. Castillo:AstraZeneca: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Kite: Consultancy; Mustang Bio: Consultancy; Cellectar: Consultancy, Research Funding. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal